Elpenhaler® is covered by more than a hundred (100) patents worldwide (Europe, US, Canada, China, Japan, Australia, Russia, etc.), which ensure legal coverage and protection for all the medicinal products administered using Elpenhaler®.
Elpenhaler®, along with every single medicinal product administered through it (Formopen®, Fluticapen®, Rolenium®, Pulmoton®), are brands with trademarks registered at national, European, and international level.
Elpenhaler® consists of 3 compartments, which interconnect but open individually:
· Mouthpiece with cover
· Supporting surface fpr placement of the dose
· Storage compartment containing 60 single-dose blister strips
Depending on the proprietary medicinal product, the package may include an extra storage compartment containing 60 single-dose blister strips. Each package contains a total of 60 or 120 doses.
Elpenhaler® inhalers are designed to feature one or two slots on the blister strip surface, depending on whether the single-dose blister strip of the drug to administer includes one or two blister pods.
The double-blister Elpenhaler® is the second version of the device, designed for simultaneous administration of two active substances separately, as opposed to a mixture.
In this case, active substances are stored in separate blister pods, so that they are only mixed when the patient breathes in.
Blister strip
A single-dose blister strip includes:
- Two easily detachable aluminum foil strips
- One or two blister pods containing the medicine dose(s)
- A small hole ensuring proper installation of the blister strip on the designated surface
Blister strip surface
The proprietary design of the blister strip surface includes the following components:
- A connection point where the strip is secured
- One or two cavities to accommodate the blister pod(s)
- Two guides securing proper placement of the strip on the surface
Elpenhaler® is Elpen’s proprietary patent protected, breath activated dry powder inhaler (DPI), for the administration of multiple single doses of medicine to treat patients suffering of asthma or COPD (Lung Obstructive diseases).
The Elpenhaler® is a reliable and versatile inhalation platform for the respiratory administration of numerous Active Pharmaceutical Ingredients (APIs).
The drugs to be administered are included in the product package, in the form of specially designed, simple-dose blister packs.
Click HERE to print the Elpenhaler® instructions for use
The Elpenhaler® inhaler was developed for the administration of medicines preventing and treating diseases of the lower respiratory tract (Asthma and Chronic Obstructive Pulmonary Disease).
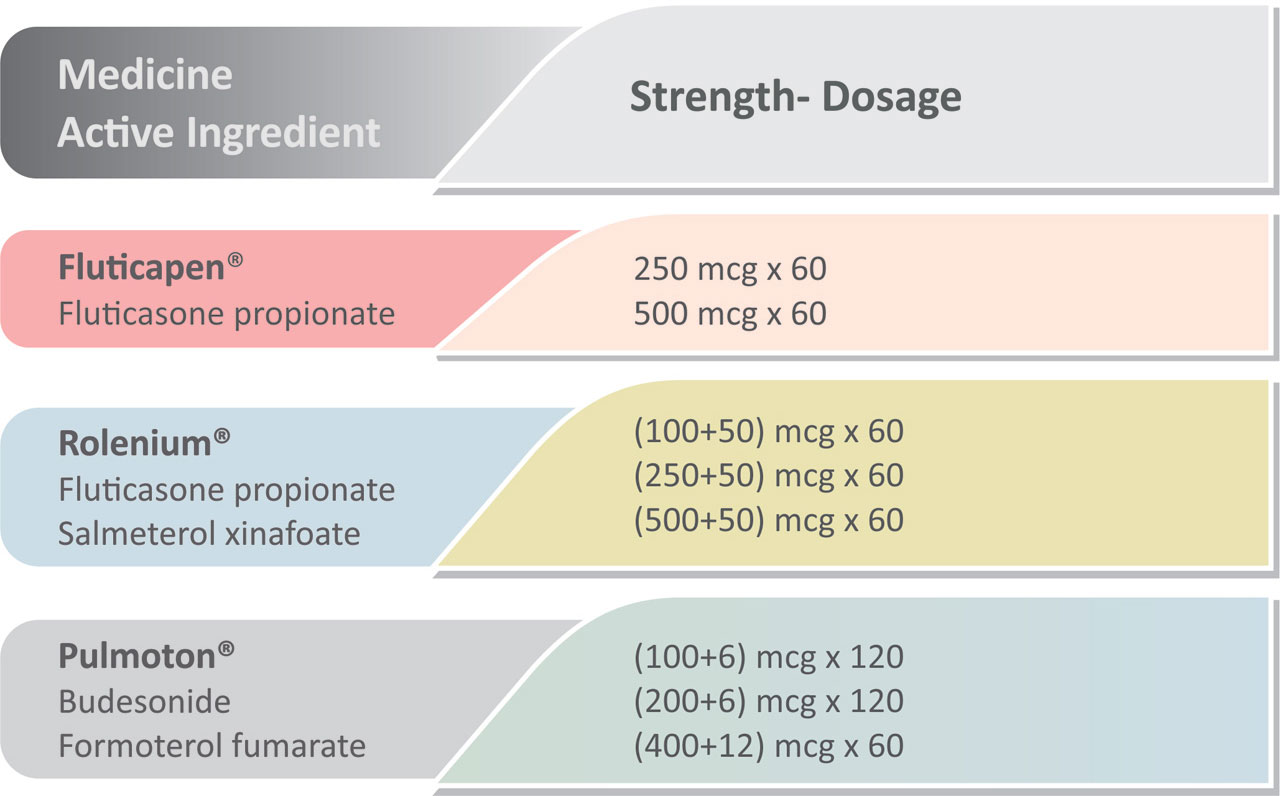
Elpenhaler® is used for the administration of the following medicines:
Rolenium® Elpenhaler®, an original product developed by ELPEN, has received administration approval for diseases of the lower respiratory tract in 34 countries in Europe.
Pulmoton® Elpenhaler® has received administration approval for diseases of the lower respiratory tract in 11 countries in Europe.

An extensive clinical trial programme is currently in progress for all products administered with Elpenhaler®; the programme includes Phase ΙΙΙ and IV studies.
The clinical development programme is based on the latest EMEA/CPMP guidelines.
However, the ever-changing pharmaceuticals industry requires constant monitoring of every single event, to ensure that the clinical documentation for all products administered with Elpenhaler® corresponds to the latest data and that the product may receive approval in as many countries as possible.
According to international guidelines and the responsibility and transparency policy implemented by ELPEN in the context of clinical trials, all relevant communications are transparent.